The Problem of Unsolved Cases
While our knowledge of human genetics has grown significantly, many gaps remain in our understanding of genes and their contribution to human disease.1-3
We have a lot to learn:
• Only about 16% of all the genes in the human body have an established disease association.
• The genetic cause is known for only half (46%) of clinically defined Mendelian conditions.
• Current technologies only diagnose approximately 25-35% of people with a suspected Mendelian condition, even when exome and genome sequencing is pursued.
Luckily, we’re discovering more every day:
• It’s estimated that a new gene-disease relationship is characterized every 2 days.
• About one-third of positive exome results are in genes characterized in the last few years.
What’s clear from this evidence is that the discovery of gene-disease relationships should remain a priority for the genomics community, including commercial laboratories, as it can directly impact the clinical utility of genetic testing. Defining new genetic causes of disease can’t be done in silos. It's essential to bring together stakeholders—researchers, clinicians, patients and families, and diagnostic laboratories—so that we can harness the power of our collective knowledge to push discovery forward, especially for rare and ultrarare conditions.
What is GeneMatcher?
GeneMatcher is a freely accessible and user-friendly website developed in 2015 as part of the Centers for Mendelian Genomics network.4 According to the website, “GeneMatcher is designed to enable connections between patients, their families, clinicians and researchers from around the world who share an interest in the same gene or genes. The principal goal for making GeneMatcher available is to help solve 'unsolved' exomes.” GeneMatcher is part of the Matchmaker Exchange5, a network designed to “facilitate the matching of cases with similar phenotypic and genotypic profiles (matchmaking) through standardized application programming interfaces (APIs) and procedural conventions.” Matchmaker Exchange is a truly global effort, with more than 12,000 contributors from more than 100 countries submitting >120,000 cases to date.
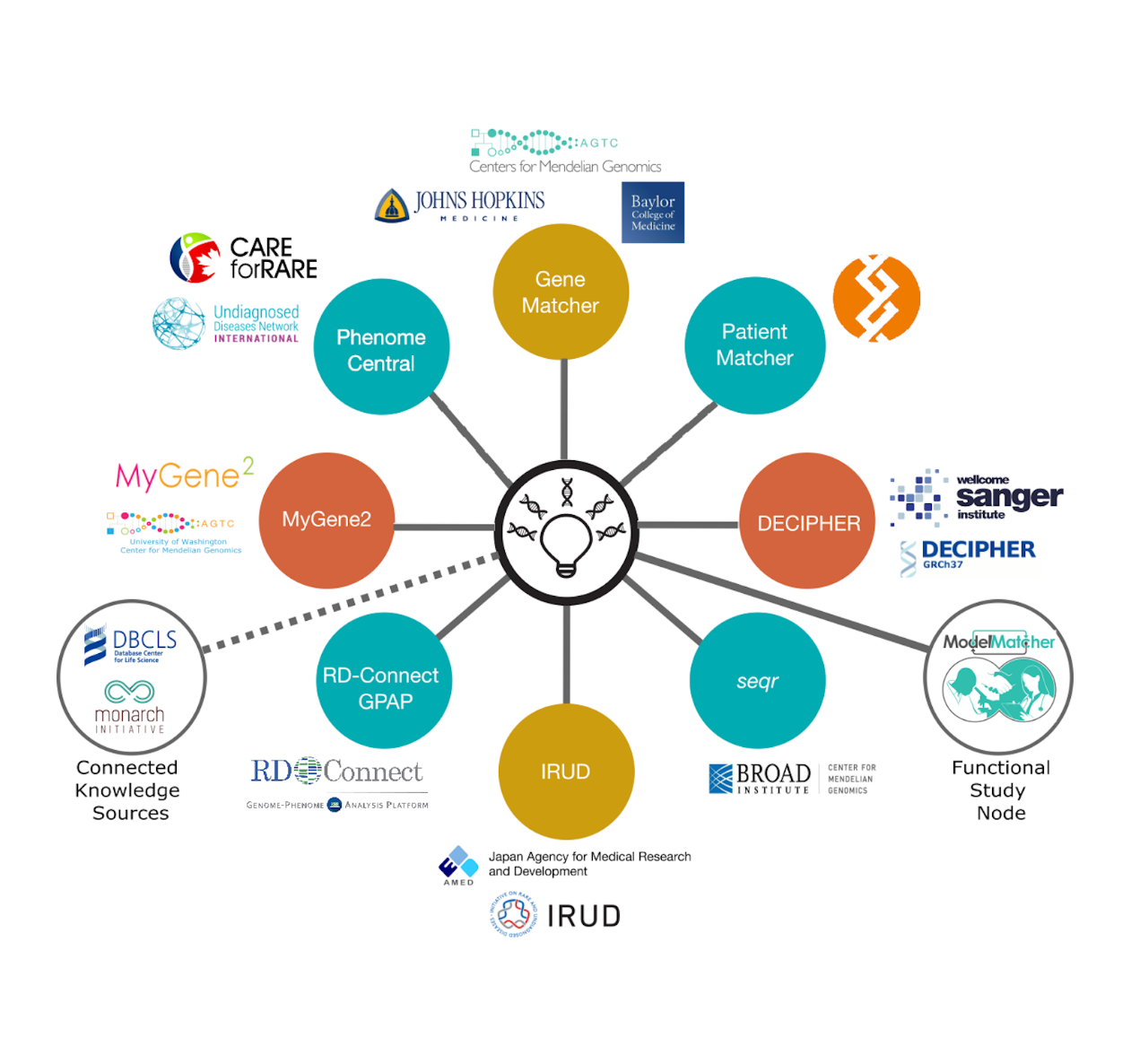
Importantly, using GeneMatcher is easy. A new entry takes less than 5 minutes and includes entering the gene of interest, inheritance if known, details of the variant (like loss-of-function or missense) and clinical features. There are appropriate privacy safeguards in place. Once you create an entry, matching is initiated, and you receive an email back to notify you if there are any matches. The match list provides users with a list of others who have submitted similar entries for that gene. Typically, these matches start open, collaborative discussions amongst the submitters to determine if there is a match in terms of the type of inheritance and phenotype. Case series may emerge with a more formal collection of clinical features, variant details, mechanism of disease, etc. Publications can develop, and GeneMatcher only asks that they are cited if a publication results from a match.
Every exome run has the potential to not only provide a much sought-after answer for that individual family but also fuel the discovery of new gene-disease relationships, which can end the hunt for a diagnosis for many others around the world. GeneMatcher centralizes this process and makes discoveries more streamlined and feasible.
Why Should Diagnostic Labs Like Ambry Contribute to GeneMatcher?
Diagnostic laboratories are in a unique position to contribute to gene-disease discovery efforts for several reasons:
1. Case volumes. Labs that perform a high volume of exome or genome sequencing will have a large dataset of variants in uncharacterized genes and, therefore, can screen these large cohorts for patients with rare variants in a gene and overlapping clinical features. Diagnostic labs may even be the first to note an overlap in genetic variants and clinical features across multiple patients.
2. Bridging stakeholders. Labs are also well-positioned to help clinicians, researchers and patients connect. If a candidate gene is identified, we reach out to clinicians to share information on research efforts and help to facilitate appropriate consent from the patient is obtained before publication.
3. Facilitating additional testing or sample transfers. Labs may have residual samples available for further testing, offer resources for confirmatory or follow-up studies of interest, or share raw data—all with appropriate releases.
4. Providing expertise for research and publication. Scientists working for commercial labs often have deep subject matter expertise and an interest in contributing to the field through publications, as well as resources available for doing so.
Benefits of Collaboration

At Ambry, we’ve been actively collaborating via GeneMatcher since 2016. In 2022, we published our 5+ year experience in a peer-reviewed article for Human Mutation.6 Ambry is unique in that we’re the only commercial laboratory with validated, published guidelines for curating and scoring gene-disease validity.7 We also have a team of scientists on staff who continuously curate the peer-reviewed literature to evaluate new evidence for gene-disease relationships. This is integrated into our reporting process, which allows us to systemically identify interesting variants in uncharacterized genes with compelling evidence for disease association and submit high-quality information to GeneMatcher.
Between March 2016 and September 2021, we submitted 246 candidates from 243 unique genes to GeneMatcher, of which 111 (45%) were clinically characterized at the time of the paper. We coauthored 104 publications delineating gene-disease relationships, including descriptions of new associations (60%), additional supportive evidence (13%), subsequent descriptive cohorts (23%), and phenotypic expansions (4%). Many of these publications were driven by GeneMatcher submissions.
We continue to invest resources in the discovery of new gene-disease relationships because it aligns with the ethos of our Ambry Classifi™ Program, which is our commitment to finding potentially life-changing answers for every patient. The outputs of this effort contribute to the entire field of genomics, which we view as a responsibility as a diagnostic laboratory. These investments also help drive improvements in our products as we can increase the diagnostic yield of exome testing, report results with higher confidence, thoughtfully design gene panels, and build service offerings like our proactive reanalysis program, Patient for Life.
Learn more
Read the publication in the special issue of Human Mutation, Matchmaker Exchange: Seven years of discovery and collaboration.
___
References
1. Bamshad MJ, Nickerson DA, Chong JX. Mendelian gene discovery: Fast and furious with no end in sight. Am J Hum Genet. 2019 Sept 5;105(3):448-455. doi: 10.1016/j.ajhg.2019.07.011.
2. Farwell Hagman KD et al. Candidate-gene criteria for clinical reporting: diagnostic exome sequencing identified altered candidate genes among 8% of patients with undiagnosed diseases. Genet Med. 2017 Feb;19(2):224-235. doi: 10.1038/gim.2016.95.
3. 100,000 Genomes Project Pilot investigators. 100,000 genome pilot on rare-disease diagnosis in healthcare – preliminary report. NEJM. 2021 Nov 11;385(20):1868-1880. doi: 10.1056/NEJMoa2035790.
4. Sobreira N, Schiettecatte F, Valle D, Hamosh A. GeneMatcher: A matching tool for connecting investigators with an interest in the same gene. Hum Mutat. 2015 Jul 29. doi: 10.1002/humu.22844.
5. Philippakis AA et al. The Matchmaker Exchange: A platform for rare disease gene discovery. Hum Mutat. 2015;36:915-921. doi: 10.1002/humu.22858.
6. Towne MC et al. Diagnostic testing laboratories are valuable partners for disease gene discovery: 5-year experience with GeneMatcher. Hum Mutat. 2022 Feb 10;43(6):772-781. doi: 10.1002/humu.24342.
7. Smith ED et al. Classification of genes: Standardized clinical validity assessment of gene-disease associations aids diagnostic exome analysis and reclassifications. Hum Mutat. 2017 May:38(5):600-608. doi: 10.1002/humu.23183.



