As we find more ways to take charge of our health, genetic testing is becoming something that many consider and, in fact, has become easier to access than ever before. An appointment with a doctor or genetic counselor is no longer required to access genetic testing – for some types of genetic tests, it’s now possible to order a test kit online, send your saliva sample right to the lab and click to get your results.
However, Direct-to-consumer (DTC) and medical-grade genetic tests are not the same. If you are considering genetic testing, it is helpful to understand some key differences between the two available types of testing to you can make an informed decision.
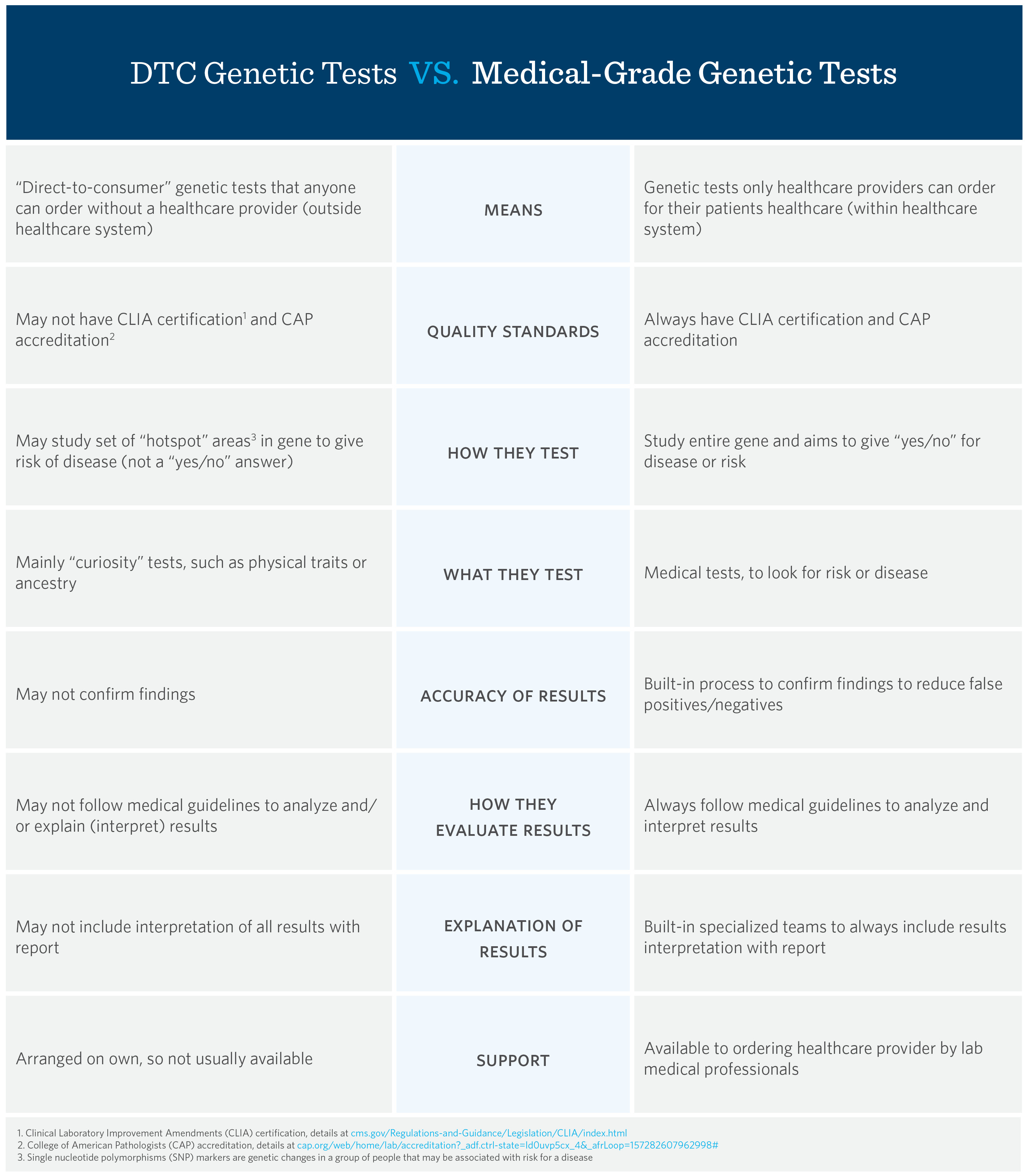
Key Difference #1: Testing Methods
DTC genetic testing companies may only look at a set of single nucleotide polymorphism (SNP) markers, common “hotspots” in genes that are associated with a particular disease, to determine one’s risk to develop a disease, which does not yield a clear “yes/no” answer.
In contrast, medical-grade genetic testing thoroughly studies the entire DNA sequence of genes known to cause a disease. It looks for tiny changes and, often, areas that are missing/extra. This is quite comprehensive and can determine if someone has a disease or is at risk to develop it. Medical-grade genetic testing aims to give a “yes/no” answer either way.
-
Bottom line: DTC testing will not usually give you a definitive answer about disease.
Key Difference #2: Quality Standards
Quality standards between DTC and medical-grade laboratories can greatly differ. Medical-grade genetic testing labs are all Clinical Laboratory Improvement Amendments (CLIA) certified1 and College of American Pathologists (CAP) accredited.2 DTC labs may not be.
These credentials mean a lab went through rigorous steps to prove they could meet established quality standards. Some DTC labs may have FDA approval to offer genetic testing, but do not have CLIA/CAP credentials. Even if a lab has FDA approval, the FDA states, “Results obtained from the tests should not be used for diagnosis or to inform treatment decisions. Users should consult a health care professional with questions or concerns about results.”3
-
Bottom line: Only results from CAP/CLIA certified labs can be used for medical diagnoses.
Key Difference #3: How Results are Handled
DTC genetic testing labs may not double-check their findings, which can lead to false positive results. Sometimes for an additional cost, they may offer “raw” data, which you are left to interpret and understand on your own.
Medical-grade labs have systems in place to confirm test results, as well as trained professionals on staff who analyze, report and explain all results according to medical guidelines in a report patients receive through your healthcare provider.
-
Bottom line: Medical-grade genetic testing gives you results that have been confirmed and carefully analyzed.
Key Difference #4: Available Support
DTC genetic testing labs typically do not have medical professionals available to answer your questions, which leaves you with the responsibility of researching a particular finding, or seeking out consultation with a healthcare professional who can interpret the results and discuss the implications with you.
Medical-grade labs have specialized professionals who will support your healthcare provider throughout the testing process and help them understand your results, which allows them to develop better health management options for you. Medical-grade labs are also increasing patients’ access to medical-grade genetic testing by partnering with healthcare professionals who deliver care in convenient ways for patients (such as telehealth).
-
Bottom line: Medical-grade genetic testing labs support your healthcare provider, who, in turn, supports you.
To learn more about what to consider before taking an at-home DNA test, click here to read the National Society of Genetic Counselors (NSGC) blog post by Brianne E. Kirkpatrick, a genetic counselor.
To learn about recent research on DTC genetic testing results, click here.
References
- Centers for Medicare & Medicaid Services. “Clinical Laboratory Improvement Amendments (CLIA)” Accessed May 2, 2017. Available from https://www.cms.gov/Regulations-and-Guidance/Legislation/CLIA/
- College of American Pathologists. “Accreditation” Accessed May 2, 2017. Available from http://www.cap.org/web/home/lab/accreditation?_adf.ctrl-state=ld0uvp5cx_4&_afrLoop=157282607962998#
- FDA News Release. “FDA allows marketing of first direct-to-consumer tests that provide genetic risk information for certain conditions.” Accessed May 5, 2017. Available at https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm551185.htm.
Resources to Learn More
- Centers for Disease Control and Prevention. Public Health Genomics: “ACCE Model Process for Evaluating Genetic Tests” Accessed May 2, 2017. Available from https://www.cdc.gov/genomics/gtesting/ACCE/index.htm
- Genetic Alliance. “Genetic Testing” Accessed May 2, 2017. Available from http://www.geneticalliance.org/advocacy/policyissues/genetictesting
- National Institutes of Health (NIH). Genetics Home Reference: “How can consumers be sure a genetic test is valid and useful?” Accessed May 2, 2017. Available from https://ghr.nlm.nih.gov/primer/testing/validtest
- World Health Organization. “Quality & Safety in Genetic Testing: An Emerging Concern.” Accessed May 20, 2017. Available from http://www.who.int/genomics/policy/quality_safety/en/index1.html



