We present impactful research to move the science of genetics forward at tradeshow and conferences throughout the year.
Check out some of the research, separated by category below, that we brought to this year’s National Society of Genetic Counselor’s annual meeting. Read about the contributions from our lead researcher Zoe Powis and the Ambry research that won ‘Poster of the Show’ here.

Multi-Gene Panel Testing Results
Jackson, M., et al: Multi-gene Panel Testing for Prostate Cancer: What Have We Learned So Far?
Currently, genetic testing guidelines for prostate cancer are limited to BRCA1/2, however many genes have been implicated in association with hereditary prostate cancer. The research examined data from men diagnosed with prostate cancer who underwent any multi-gene panel test (MGPT) prior to the offering of aa prostate-specific panel (ProstateNext) and any patient who underwent ProstateNext testing after it was available.
The study compared these two groups to look for similarities and differences. Similarities included the mutation detection rate (14.2% on other MGPT vs. 14.7% on ProstateNext) and the gene distribution of positive test results (BRCA1/2, ATM, CHEK2, and PALB2 were among most frequent). Differences were also observed in the gene distribution - for instance, HOXB13 was newly identified on ProstateNext and Lynch syndrome genes and TP53 were not identified on ProstateNext. Importantly, 5% of positive patients did not meet NCCN® testing criteria for BRCA1/2, this finding may contribute to the expansion of NCCN® guidelines in the future.
Paudyal, T., et al: Results of multi-gene panel testing in a gastric cancer cohort: it’s not all CDH1
This poster described the results of multi-gene panel testing and the mutation spectrum observed among patients with a personal history of gastric cancer (GC). Although hereditary GC is primarily thought to be associated with CDH1, it was interesting to find genes like ATM and MLH1 to be associated with an increased risk for GC. A number of attendees said that they had patients with gastric cancer who had mutation on genes that we wouldn’t usually expect, such as FLCN or BAP1 and were glad to see they were not the only ones with such patients.
Genetic Testing Processes
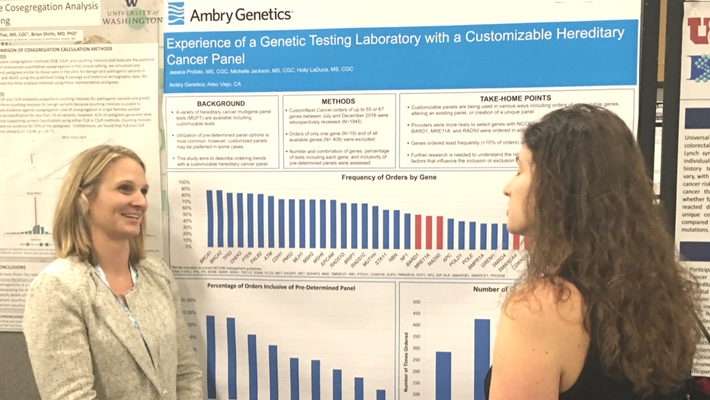
Profato, J., et al: Experience of a Genetic Testing Laboratory with a Customizable Hereditary Cancer Panel
The data presented at NSGC described the ordering trends of Ambry’s customizable hereditary cancer panel, CustomNext-Cancer. There are a variety of pre-determined multigene panel tests currently available, but many healthcare professionals and especially genetic counselors, are opting to use customizable panels. There is currently limited knowledge about how and why these panels are being ordered. Our data shows that CustomNext-Cancer is being used in a variety of ways, including orders of all available genes, tailoring an existing panel by adding or removing genes, or the creation of a completely unique test.
Genes with NCCN® management guidelines were more likely to be ordered; however, BARD1, MRE11A, and RAD50, which do not have currently available guidelines, were ordered in >50% of cases. This may suggest that providers are less comfortable ordering genes without management guidelines. Genes associated with rare conditions/cancers, like brain tumors, were ordered less frequently (<10% of cases), which may suggest that gene content is being tailored to family history.
Further research is needed to better understand the rationale for pursuing custom panels and factors that influence gene selection. This information will help to make sure that the best testing options are made available to providers and their patients.
Wetmer, E., et al: Up-front vs. post-reporting parental co-segregation analysis: Is one approach superior?
In neurological genetic testing, 40% of variants of unknown significance can be explained when both of the patient’s parents are tested for the variant(s). Based on an audience poll at the National Society of Genetic Counselors meeting, genetic counselors preferred to have parents tested at the same time as the patient and to receive a full report with results from all family members. However, this requires both parents to be available at the clinic appointment, which can be difficult. Therefore, providing the additional option to test parents after the patient’s results are complete allows for more parental participation and increases the chance for useful results.
Wayburn, B., et al: Gene Characterization: A scientific approach to multi-gene panel testing design
Clinical validity is a standard that characterizes the strength of a gene-disease relationship based on various lines of evidence. Assessment of Ambry’s clinical validity scoring system using data from Ambry’s cardiology testing menu showed that genes with “limited” clinical validity returned no positive pathogenic findings, yet added to the overall VUS rate of the panel.
Attendees viewing the poster, particularly those who work in cardiology settings, agreed with the data and appreciated the idea of building smarter panels, while still having the option for large expanded panels, such as CardioNext, that include these “limited evidence” genes.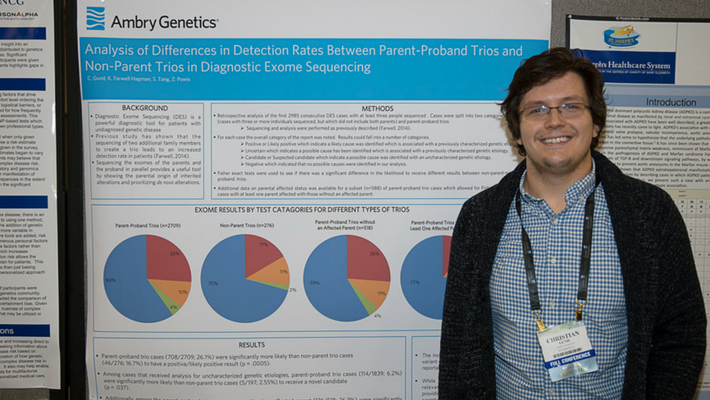
Gund, C., et al: Analysis of differences in detection rates between parent-proband trios and non-parent-trios in Diagnostic Exome Sequencing.
This study examined results from our first 2985 consecutive DES cases where at least three people were sequenced. It compared the results received between parent-proband trios and trios which did not include both parents. Parent-proband trios were significantly more likely than non-parent cases to have a positive or likely positive result. Also, the parent proband cases were significantly more likely to receive a novel candidate with an uncharacterized genetic etiology.
Additionally, this cohort highlights the difficulty of analysis in parent proband trios where at least one parent is affected. These cases were found to be significantly less likely to receive a positive or likely positive result than those with two unaffected parents. This study shows that the increased utility of parent-proband trios leads to a greater rate of positive results. Thus, while Ambry always recommends sending in samples from as many relevant family members as possible, families considering ordering exome analysis should seek to test samples from both parents whenever possible.
This research presented results from an important project to reduce conflicts in variant interpretation between laboratories. ClinGen variant interpretations were compared among 42 clinical laboratories submitted to ClinVar to identify outlier classifications. The vast majority of variant classifications were in agreement between laboratories; however 2.5% (1 in 40) of shared variants between labs had medically significant differences, potentially impacting medical management.
Preliminary results of initial re-evaluation of alterations exceeded expectations, with 76% of cardiovascular and 58% of hereditary cancer alterations reviewed resulted in reclassification or updating ClinVar. Upon completion of initial re-evaluations, it is anticipated that at least an additional 33% of initial conflicts will be resolved by sharing internal data between laboratories. The ultimate goal of the project is to review all classification conflicts between clinical laboratories, anticipating resolution of at least 80% of prior variant interpretation conflicts between laboratories. By helping the research community move towards more consistent variant interpretations, we can ultimately improve the care of patients who may be atrisk for genetic disorders.
Disease Research
Allen, K., et al: Family Communication (FC) of Test Results among an Unselected Pancreatic Ductal Adenocarcinoma (PC) Cohort who Underwent Germline Genetic Testing
Pancreatic cancer is among the deadliest cancer diagnoses, 92% of those diagnosed have a 5-year mortality rate, which emphasizes the importance of screening and risk reduction among those who may be susceptible. Our pancreatic cancer collaborative study suggests that in up to 12% of diagnoses of pancreatic cancer mutations, cancer susceptibility genes may play a significant role.
Identifying these germline mutations in affected individuals offers an important opportunity for testing in relatives who may carry a mutation. In fact, the research showed that the majority of affected individuals identified as mutation carriers share genetic testing results with family members. This research highlights the importance of future studies concerning cascade testing so that high risk individuals who may benefit from increased surveillance and prevention practices may be readily identified.
Pritzlaff, M., et al: APC I1307K Homozygotes: Classic FAP or Something Else?
When a clinician receives an I1307K homozygous result for an Ashkenazi Jewish individual, there is uncertainty. Will this patient be more likely to have classic FAP since they have two mutations, or will they present more like I1307K heterozygotes?
This research set out to answer this question by looking at the largest group of I1307K homozygotes reported to date. We evaluated the colorectal cancer risk of homozygotes compared to heterozygotes and negative Ashkenazi Jewish individuals tested at Ambry. While we did find a trend towards increased risk for colorectal cancer in homozygotes compared to heterozygotes (OR 4.5 p=0.1 95% CI [0.4, 33.3]) and compared to negative individuals (OR 6.5 p=0.06 95% CI [0.6, 45.2]), the trends did not reach statistical significance.
It appears that these individuals do not present with extra-intestinal manifestations of FAP or with classic polyposis, and it also appears that they have an increased risk for colorectal cancer, similar to (or possibly higher than) I1307K heterozygotes. As more homozygotes are identified, future studies with targeted examinations will provide even greater clarification of this phenotype and the colorectal cancer risk.
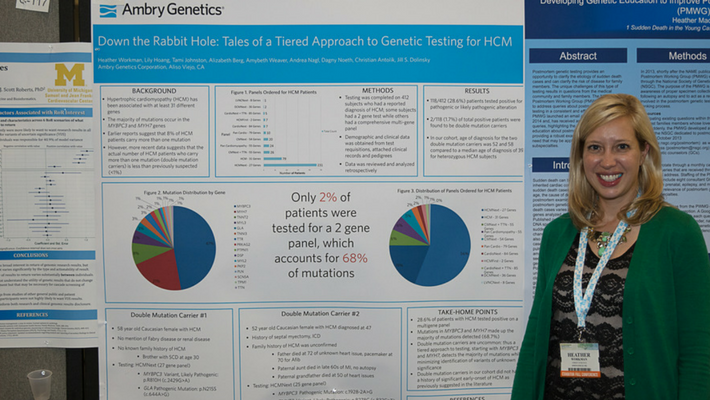
Workman, H., et al: Down the Rabbit Holes; Tales of a Tiered Approach to Genetic Testing for HCM
The presented data examined our hypertrophic cardiomyopathy (HCM) panels by examining all the subjects referred for genetic testing to detect a diagnosis of HCM, and which panels were ordered for those cases.
Most providers chose a comprehensive panel for HCM; however, Ambry also offers a tiered approach to testing which first looks at the MYH7 and MYBPC3 genes, (which account for 68% of mutations detected at Ambry), and then reflexes to a more comprehensive panel with 25 additional genes if a mutation is not found in the first 2 genes.
The resulting data detected double mutations in 1.7% of total positive patients in our cohort, which supports Ambry’s tiered approach. The poster generated a lot of discussion amongst genetic counselors about a targeted vs. comprehensive approach to testing.



