
Did you know that more than 1 in 4 of those with Lynch syndrome (LS) are missed by current genetic testing guidelines? New research from Ambry Genetics and Ohio State University of nearly 35,000 patients will change how the genetics community thinks about genetic testing strategies, lifetime cancer risks, and medical management for people with LS.1 The study described patients with mismatch repair (MMR) gene mutations tested using multigene panels and compared results to previous studies on LS. It also included an analysis of whether study patients would meet existing LS testing guidelines.
Read the full research paper in Journal of Clinical Oncology.
Background
Lynch syndrome (LS) is a hereditary cancer syndrome historically associated with high lifetime risks for colorectal cancer (CRC) and endometrial cancer (EC), although other cancers are also part of the syndrome. LS is estimated to occur in 1 in 440 people2, but recent research suggests this could be as high as 1 in 279 – which would make LS the most common hereditary cancer syndrome.3
Multigene panels are increasingly utilized for LS; their results can impact risk assessment and direct medical management, often following published guidelines.4Largest study of people tested with multigene panels for Lynch syndrome, which can change patient care.
However, many current LS guidelines are based on evidence biased toward colorectal cancer (CRC) and/or endometrial cancer (EC), and many did not analyze all LS genes.5-10 Basing LS genetic testing on a patient meeting Amsterdam criteria or Bethesda guidelines may miss as many as 72% and 27% of those with LS, respectively.8 Screening for LS with microsatellite instability (MSI) and/or immunohistochemistry (IHC) for the MMR proteins may miss 13-23%.8
Study in Brief
Ambry and Ohio State University researchers reviewed genetic test results and clinical information on 34,980 patients who underwent multigene panel testing that included the 5 MMR genes and EPCAM at Ambry, from March 2012 to June 2015.1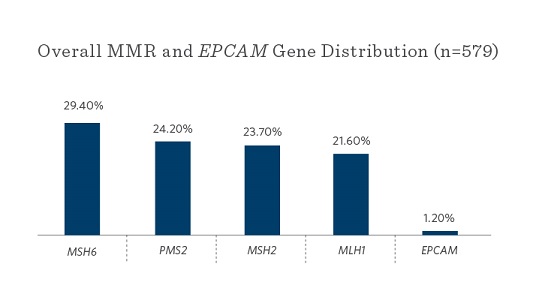
Key Results
- Overall, 27.3% of those with LS are missed by current genetic testing guidelines
- >25% of LS gene mutation carriers presented with breast or ovarian cancer as their first primary cancer
- MSH6 and PMS2 mutations were most commonly seen
How Current Genetic Testing Guidelines Stacked Up4,11
- 72.7% of patients met NCCN® guidelines for LS testing
- 58.9% met the NCCN® guidelines for BRCA1/2 testing
- 36.7% met both NCCN® guidelines
- 22.2% met only NCCN® guidelines for BRCA1/2 testing
- 5.1% did not meet any testing criteria (LS or BRCA1/2 testing)
- MSH6 and PMS2 mutation carriers were significantly more likely to just have breast cancer and only meet BRCA1/2 testing criteria
Points for Your Practice
- This study reports on the largest group of individuals tested for and diagnosed with LS through multigene panel testing
- Current genetic testing guidelines may miss more than 1 in 4 people with LS
- Current understanding of genes implicated in LS and lifetime cancer risks is incomplete
- MSH6 and PMS2 mutations were most commonly found (as compared to MLH1 and MSH2 in previous studies)
- >20% of MSH6 and PMS2 mutation carriers had a hereditary breast-ovarian cancer (HBOC) clinical presentation
To learn more about Ambry’s cancer panel testing options for Lynch syndrome, click here.
To learn more about Lynch syndrome on Ambry’s website for patients and families, click here.
References
- Espenschied C, et al. Multi-gene panel testing provides a new perspective on Lynch syndrome. J Clin Oncol. April 2017 [Epub ahead of print].
- Chen S, et al. Prediction of germline mutations and cancer risk in the Lynch syndrome. JAMA. 2006 Sep 27;296(12):1479-87.
- Win AK, et al. Prevalence and penetrance of major genes and polygenes for colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2017 Mar;26(3):404-412.
- National Comprehensive Cancer Network®. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Genetic/Familial High-Risk Assessment: Colorectal. Version 2.2016. Accessed April 25, 2017. Available from https://www.nccn.org
- Hampel H, et al. Screening for Lynch syndrome (hereditary nonpolyposis colorectal cancer) among endometrial cancer patients. Cancer Res. 2006 Aug 1;66(15):7810-7.
- Hampel H, et al. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol. 2008 Dec 10;26(35):5783-8.
- Bonadona V, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA. 2011 Jun 8;305(22):2304-10.
- Palomaki GE, et al. EGAPP supplementary evidence review: DNA testing strategies aimed at reducing morbidity and mortality from Lynch syndrome. Genet Med. 2009 Jan;11(1):42-65.
- Moreira L, et al. Identification of Lynch syndrome among patients with colorectal cancer. JAMA. 2012 Oct 17;308(15):1555-65.
- Moller P, et al. Cancer incidence and survival in Lynch syndrome patients receiving colonoscopic and gynaecological surveillance: first report from the prospective Lynch syndrome database. Gut. 2017 Mar;66(3):464-472.
- National Comprehensive Cancer Network®. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Genetic/Familial High-Risk Assessment: Breast and Ovarian. Version 2.2017. Accessed April 25, 2017. Available from https://www.nccn.org



