At Ambry, we’re continuing our track record of scientific innovation and discoveries with our latest hereditary cancer testing menu enhancements and reporting updates—powered by Ambry Classifi®.
Our peer-reviewed, published gene-disease validity (GDV) scheme enables the enhanced assessment and characterization of RPS20 for colorectal cancer predisposition.
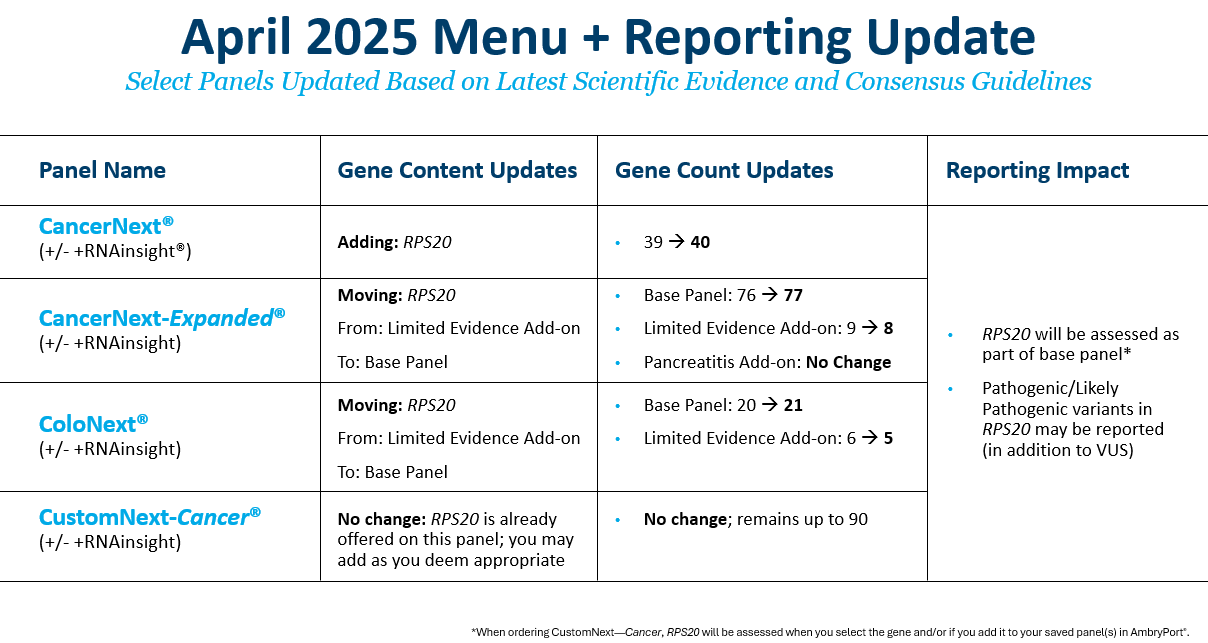
We’ve updated some of our tests to include RPS20 in the panel (CancerNext®) and base panels (CancerNext—Expanded® and ColoNext®). We’ve made this change for two key reasons:
1. Our data has upgraded this gene-disease relationship from limited to moderate GDV. The available evidence has been reviewed extensively by our internal experts, affectionately called the Gene Team.
2. Medical management guidelines exist for this gene.
This change means that reportable variants could be “likely pathogenic” and “pathogenic” (in addition to “variant of uncertain significance (VUS)”) for this gene.
To learn more about the science and data that drove this menu enhancement, check out this poster from the 2025 ACMG Annual Clinical Genetics Meeting, which earned a “Top 20” recognition.
What to know about ordering:
- Menu enhancements are automatic across digital ordering sources: AmbryPort®, CARE®, Progeny®, and integrated Electronic Health Records (EHRs).
- If you use paper Test Requisition Forms (TRFs), please ask your Ambry Account Executive (AE) for updated paper TRFs.
- If you order CustomNext—Cancer® and use AmbryPort to place your orders:
o For customers with saved panels, click on the “Saved Panels” tab and recreate your favorite panels and add RPS20 if you wish to add it to a saved panel.
o For customers who build their panels by indication and gene disease validity, please note that RPS20 has moved from the “Limited GI” grouping to the “GI” grouping (which includes moderate and above GDV)
Key Resources:
- Want to learn more about our enhanced panels, including gene content, test descriptions, and more? Click on the following:
o ColoNext
-
Need a quick and simple way to update your clinic notes? Click here, select “Gene List”, and then “Copy Gene List” for CancerNext, CancerNext—Expanded, and/or ColoNext. Then, paste the genes into your notes.
-
Looking for the latest TRFs? Click here.
If you have questions, please contact your Ambry AE. Your local Ambry Genomic Science Liaison (GSL) is available if clinical support would be helpful. You may also contact Client Solutions at (949) 900-5500, info@ambrygen.com, or via our Messages feature within AmbryPort.
Setting the Standard in Genetic Testing
We invest in the expertise needed for ongoing evaluation of new and existing gene-disease associations to drive improvements in variant classification, smart panel design, and proactive patient care. We’re proud of our uncompromising commitment to setting the standard in genetic testing for over 25 years.



