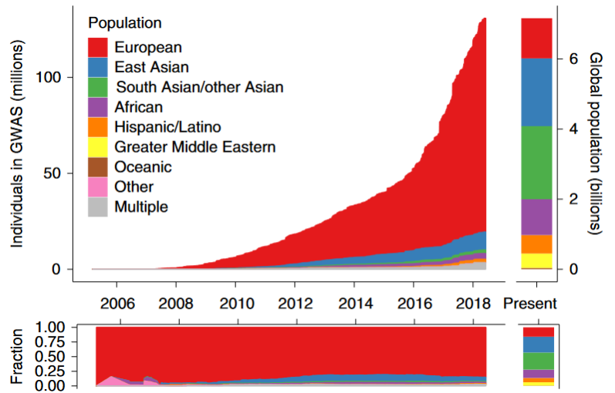
We know there are healthcare disparities among racial and ethnic groups; these disparities also impact genetic testing. Research and clinical studies have lacked diverse representation and have been predominantly composed of people with European ancestry. As rates of testing have rapidly increased, this gap has only widened. This means much of the data available doesn’t tell the full story. Nondiverse data lacks genetic variation, and can skew population frequencies, among other issues.
Using nondiverse data for clinical genetic testing interpretation directly impacts populations who are already historically poorly represented. Some minority groups in the US have higher rates of VUS (variant of uncertain significance) and lower rates of diagnostic test results on panel testing. One study even found that some patients of African ancestry received test results misclassified as pathogenic, due to a lack of representative data. These disparities are in addition to known barriers to accessing genetics services, including decreased referral rates and structural and institutional racism. These findings suggest a problem of equity in genetic testing and indicate the need for further research so these disparities can be addressed and reduced.
This study looked specifically at outcomes of exome sequencing. Exome sequencing is used for a wide range of clinical indications, and once exome sequencing is performed it can be reanalyzed. This is helpful because results can be updated when new genes are characterized or when variant interpretations change with new information. Emerging evidence shows that exome sequencing outcomes can see similar racial and ethnic disparities as other genetic tests. Therefore, exome reanalysis represents a step to potentially reduce such disparities by updating and correcting reports over time.
An exome reanalysis must be initiated—either by the ordering provider, the clinical laboratory, or as a result of family studies. The ordering provider can simply request reanalysis from the laboratory, who will review the exome data for that specific patient. Ambry Genetics employs a different strategy of proactive reanalysis. Our team of scientists is constantly reviewing new variant- and gene-level evidence in the literature. When they identify new information that could impact variant interpretation, data from all patients with a relevant variant are reviewed to identify which require an updated variant classification. This is provided at no additional cost through our Patient for Life program.
Key Results of the Study
We analyzed 10,321 patients, representing 10 years of exome data at a clinical laboratory. Almost 2,000 patients received 2,230 reanalyses, as reanalysis can happen more than once for one patient. 799 of these 2,230 reanalyses resulted in reclassification, meaning that a variant was identified that needed an updated classification.
Original Exome Outcomes
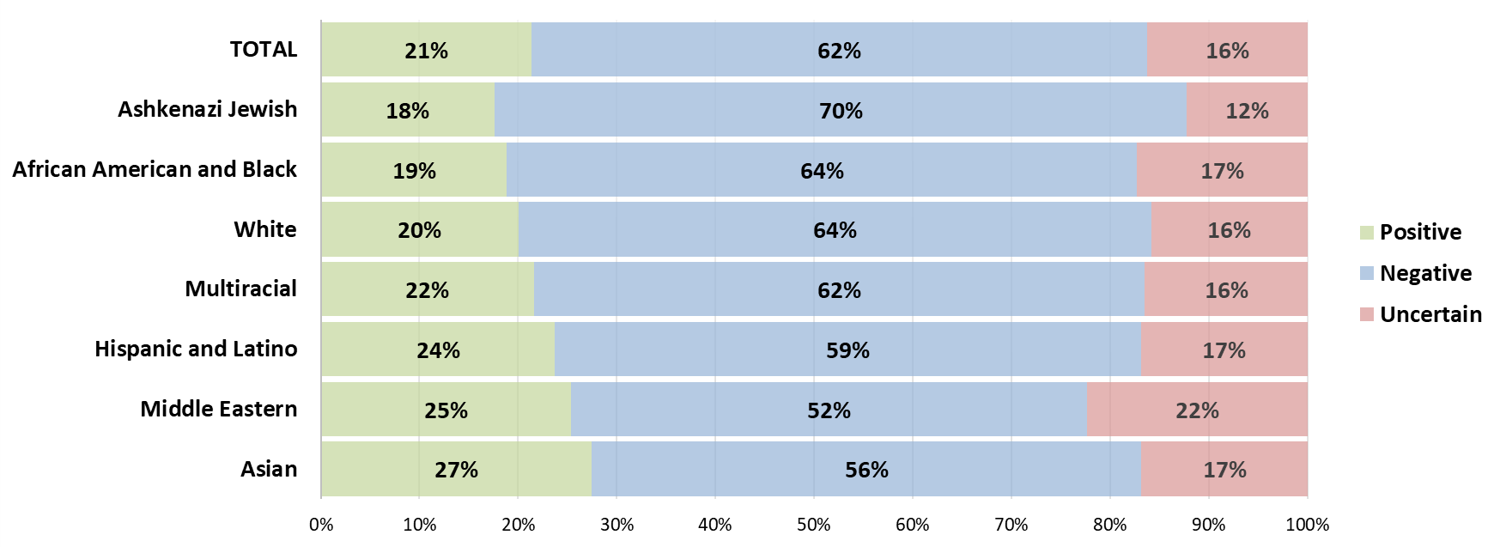
Race and ethnicity were found to be associated with the rates of positive, negative, and uncertain exome results.
Reanalysis Rates
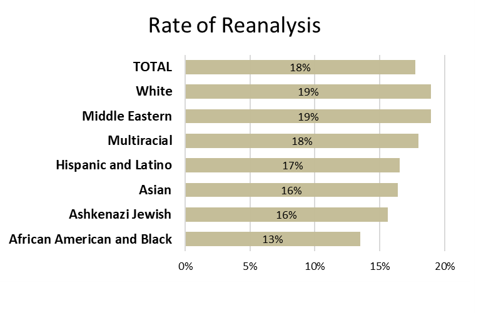
This study revealed that the likelihood of a patient receiving reanalysis is highly correlated with their race or ethnicity. Compared to the White group, the African American and Black group was significantly less likely to receive reanalysis and had a lower rate of provider-initiated reanalysis.
 Reanalysis Impact
Reanalysis Impact
Of those who would eventually receive exome reanalysis, their original rate of positive results was 6%, with a high rate of VUS. However, once these patients received their reanalyses, each group, regardless of race or ethnicity, saw a great increase in positive results, with the total cohort positive rate rising to 24%. The patients who received reanalysis needed it and those diagnoses achieved with reanalysis would likely have remained missed otherwise.
Reclassification Rates
Rate of reclassification was also impacted by patient race and ethnicity. Interestingly, some of the groups with high rates of reanalysis have lower rates of reclassification. For example, the White group had the highest reanalysis rate, but has the lowest reclassification rate.
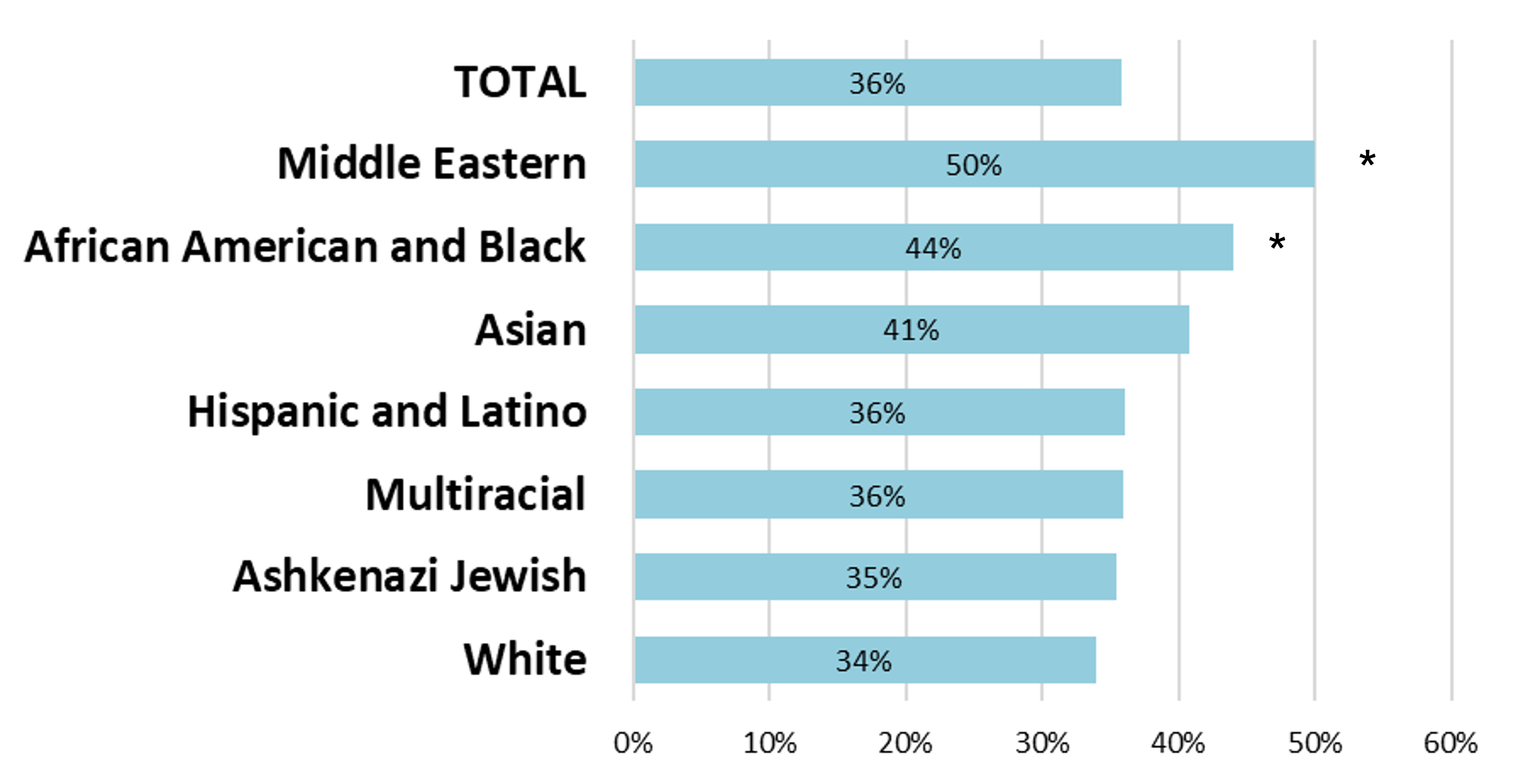 In conclusion, exome reanalysis increased diagnostic yield for all racial and ethnic groups analyzed. However, one group stood out. Data show that patients in the African American and Black group were consistently left behind in exome testing, with the second lowest diagnostic yield, the lowest rate of reanalysis, quite significantly, and the second lowest rate of provider-initiated reanalysis. However, this group had the second highest rate of reclassification, showing that some of the people most in need of a reanalysis were the least likely to receive it. But when performed, it was highly productive.
In conclusion, exome reanalysis increased diagnostic yield for all racial and ethnic groups analyzed. However, one group stood out. Data show that patients in the African American and Black group were consistently left behind in exome testing, with the second lowest diagnostic yield, the lowest rate of reanalysis, quite significantly, and the second lowest rate of provider-initiated reanalysis. However, this group had the second highest rate of reclassification, showing that some of the people most in need of a reanalysis were the least likely to receive it. But when performed, it was highly productive.
Lastly, our study demonstrates that proactive, laboratory-initiated reanalysis as offered through the Patient for Life program can help reduce some of these disparities. By skipping the need for an ordering provider to request reanalysis, patients can receive the reclassifications they need to increase equity and utility of exome sequencing. Patient for Life ensures equitable access to new diagnostic information.



