As a certified genetic counselor who spent eleven years on the National Comprehensive Cancer Network® (NCCN®)'s committee for the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for breast, ovarian, and pancreatic cancers, I've witnessed firsthand the challenges clinicians face in accurately identifying patients who meet criteria for genetic testing. Today, I'm excited to share validation results for Ambry's CARE Program® (CARE), recently published in the Journal of the National Comprehensive Cancer Network.
The Challenge: Applying Complex Guidelines at Scale
Despite increased awareness of hereditary cancer predisposition, and the recommendations of numerous scientific and medical organizations that clinicians should strive to identify at-risk patients, most are not being identified. One study found that 82% of individuals with family histories suggestive of hereditary breast and ovarian cancer had no evidence of prior genetic testing.1 Other studies show that over 95% of individuals with Lynch syndrome (the most common hereditary colon cancer syndrome) pathogenic variants remain unaware of their condition.2,3
The identification of at-risk patients begins with the collection of accurate personal and family history during clinical visits. However, providers often have limited time available for this and may be unfamiliar with guidelines on who qualifies for testing, which highlights the need for other approaches. One approach has been the development of digital tools, which have been shown to successfully reduce consultation time while improving documentation, workflows, quality of care, patient safety, communication, and clinical decision support compared with paper documentation and in-person history collection.4,5,6
The Ambry CARE Program®
To this end, Ambry designed the Comprehensive Assessment Risk and Education Program (“CARE”). This program uses digital tools to streamline medical and family history collection, then automates the application of select NCCN Guidelines® to identify patients who qualify for hereditary cancer testing.7,8 The program also provides a breast cancer risk assessment using the Tyrer-Cuzick model, which enables clinicians to identify patients who qualify for increased breast cancer screening. CARE supports patients through the process by offering education and connection to third-party genetic counseling at no additional cost.
CARE was designed to identify patients meeting NCCN Guidelines testing criteria for the most common hereditary cancer indications, including hereditary predisposition to breast, ovarian, pancreatic, and prostate cancers; Lynch syndrome; familial adenomatous polyposis and MUTYH-associated polyposis.7,8 These indications represent over 90% of patients referred to Ambry for genetic testing. CARE was not designed to assess all hereditary cancer indications and does not replace the clinical judgment of genetic counselors or healthcare providers. CARE licenses the NCCN Guidelines and is tested by the NCCN to ensure compliance with their latest guidelines, and is revised as guideline updates are released.
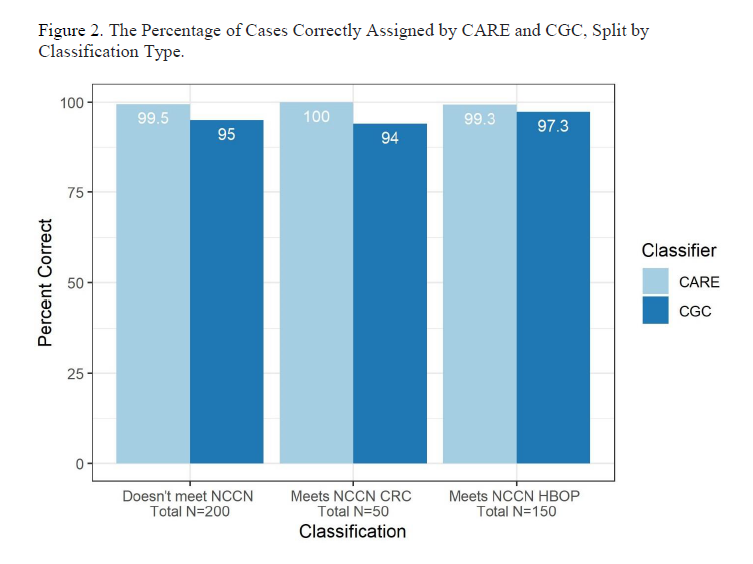
Our Validation Approach
Any model, however well designed, should be validated to show that it does what it was designed to do, which in this case is to accurately identify patients at increased risk for specific hereditary cancer indications. Thus, we put CARE through two rounds of validation.
First was an internal validation done as part of the development of the model, in which 1307 theoretical patient histories were developed that either met or did not meet criteria for genetic testing based on the versions of the time of NCCN Guidelines for Genetic/Familial High-Risk Assessment: Breast, Ovarian, Pancreatic, and Prostate7 (913 cases) and for Genetic/Familial High-Risk Assessment: Colorectal, Endometrial, and Gastric 8 (394 cases). The theoretical scenarios were then assessed independently by a team of five Ambry certified genetic counselors (CGCs) and their assessment for each case was compared with the CARE output. The consensus assessments of the GCs showed 100% concordance with CARE.
In the second, external validation, two CGCs with at least three years of recent clinical oncology experience assessed 400 real-world cases: 200 that CARE predicted did and 200 that CARE predicted did not meet NCCN Guidelines testing criteria.7,8 In this study we found that CARE accurately assessed 398 (99.5%) cases. The two cases that were incorrectly assessed were due to known limitations in the tool at the time: the inability to assess for “limited family history”; and assuming that all reported gastric cancers had diffuse histology. Of note, both limitations have since been addressed in CARE.
Why This Validation Matters for Clinicians
This study showed that CARE is as effective in identifying patients meeting NCCN Guidelines criteria for genetic testing for indications screened as experienced CGCs.7,8 It also suggests that CARE is more accurate than the paper-based tools recommended by the USPSTF.9 Accurate identification of at-risk patients is the key first step in personalizing medical management plans since high-risk patients can then be offered increased risk reduction (chemical or surgical prevention) and early detection (cancer screening) modalities, potentially leading to reduced cancer diagnoses and/or improved outcomes.
It’s important to note that our findings do not suggest a replacement for genetic counselors or other trained health professionals. The value of tools such as those offered through CARE lies in facilitating routine screening for common cancer risk within the context of busy clinical practices, which allows genetics professionals to focus on patients needing higher levels of skilled assessment and care.
As healthcare systems increasingly emphasize preventive care and early intervention, validated digital solutions like CARE will play a pivotal role in supporting hereditary cancer risk assessment at scale.
______
References
1. Kiser D, Elhanan G, Bolze A, et al. Screening Familial Risk for Hereditary Breast and Ovarian Cancer. JAMA Netw Open. Sep 3 2024; 7(9):e2435901. doi:10.1001/jamanetworkopen.2024.35901
2. Win A K, Jenkins MA, Dowty JG, et al. Prevalence and penetrance of major genes and polygenes for colorectal cancer. Cancer Epidemiology, Biomarkers & Prevention, 2017; 26(3), 404–412. https://doi.org/10.1158/1055-9965.epi-16-0693
3. Hampel H, de la Chapelle A. The search for unaffected individuals with Lynch syndrome: Do the ends justify the means? Cancer Prev Res (Phila). 2011 Jan;4(1):1-5. doi:10.1158/1940-6207.CAPR-10-0345. PMID: 21205737; PMCID: PMC3076593.
4. Wood ME, Kadlubek P, Pham TH, et al. Quality of cancer family history and referral for genetic counseling and testing among oncology practices: A pilot test of quality measuresas part of the American Society of Clinical Oncology Quality Oncology Practice Initiative. JClin Oncol. Mar 10 2014; 32(8):824-9. doi:10.1200/JCO.2013.51.4661
5. Westman J, Hampel H, Bradley T. Efficacy of a touchscreen computer based family cancer history questionnaire and subsequent cancer risk assessment. J Med Genet. May2000; 37(5):354-60. doi:10.1136/jmg.37.5.354
6. Wu RR, Himmel TL, Buchanan AH, et al. Quality of family history collection with use of a patient facing family history assessment tool. BMC Fam Pract. Feb 13 2014; 15:31. doi:10.1186/1471-2296-15-31
7. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Genetic/Familial High-Risk Assessment: Breast, Ovarian, Pancreatic, and Prostate. V.3.2025. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. Accessed [March 12, 2025]. To view the most recent and completeversion of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility fortheir application or use in any way.
8. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Genetic/Familial High-Risk Assessment: Colorectal, Endometrial,and Gastric V3.2024. © National Comprehensive Cancer Network, Inc. 2024. All rights reserved. Accessed [March 12, 2025]. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for theirapplication or use in any way.
9. Nelson HD, Pappas M, Cantor A, Haney E, Holmes R. Risk Assessment, Genetic Counseling, and Genetic Testing for BRCA-Related Cancer in Women: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. Aug 20 2019; 322(7):666-685. doi:10.1001/jama.2019.8430



