Our new case-control study shows that more than one in four people diagnosed with sarcoma have an underlying germline pathogenic variant. These findings cut across age groups and sarcoma subtypes and they challenge the idea that age at diagnosis can reliably guide who should get germline testing.
Why this matters: Sarcomas are rare and complex cancers with many subtypes and highly variable courses. Historically, germline genetic testing has been reserved for people who clearly meet criteria for syndromes like Li-Fraumeni (TP53) or Neurofibromatosis type 1, or who have very early-onset disease. Growing evidence suggests inherited cancer risk in sarcoma is broader than previously appreciated and can directly affect treatment decisions, radiation and surgical planning for patients with radiosensitive syndromes, and surveillance and preventive care for patients and their relatives through cascade testing.
What We Studied
This was a retrospective case-control analysis of 488 people with sarcoma and 2,440 matched cancer-free controls (matched 1:5 by age, sex, and ethnicity). All underwent multigene germline panel testing at Ambry Genetics between 2016 and 2024. Cases were tested on panels ranging from 1–91 genes; controls had 66–67-gene panels.
What We Found
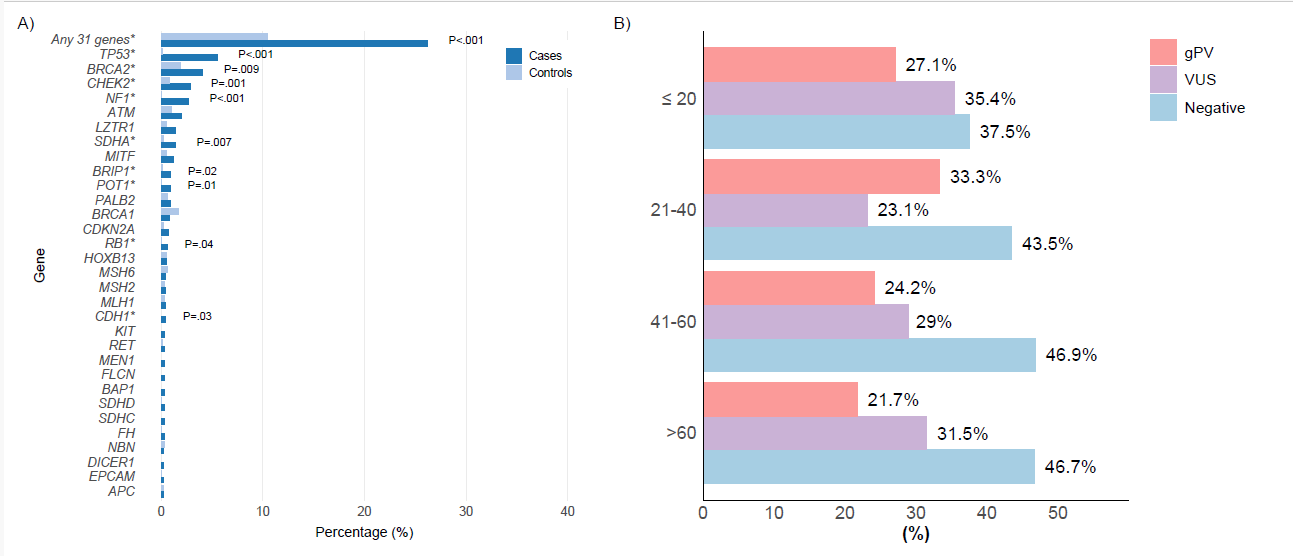
26.2% of sarcoma cases had a gPV vs 10.5% of controls (odds ratio 3.05; p < .001). Key genes enriched in sarcoma cases versus controls included: TP53 (5.5% in cases), NF1 (2.7%), BRCA2 (4.1%), CHEK2 (2.8%), SDHA (1.4%), BRIP1 (0.9%), POT1 (0.9%), RB1 (0.6%) and CDH1 (0.4%; no carriers in controls). Signals persisted even after excluding genes already linked to sarcoma risk (e.g., TP53, NF1, RB1, SDHA, POT1, BRCA2): gPVs remained higher in cases (13.6%) than controls (8.0%) (OR 1.82; p < .001), suggesting additional genes may be relevant and warrant study. 6.8% carried gPVs with potential therapy implications. Median age at sarcoma diagnosis did not differ between gPV carriers and non-carriers. gPVs were detected across all age groups, with rates exceeding 20% in every decade. Of note, nearly half of patients had more than one primary cancer. Liposarcoma was less frequent among gPV carriers; otherwise subtype distribution was similar between groups.
 What This Means for Care Today
What This Means for Care Today
Consider broader access to germline testing for people diagnosed with sarcoma. The high yield, age distribution, and direct implications for treatment and survivorship argue for multigene panel testing as part of standard workup. Unlike some cancers where “young age” strongly predicts a germline finding, sarcoma risk genes showed up broadly across ages in this study.
What This Doesn’t Prove (Important Limitations)
Selection bias is likely. Cases were people referred for genetic testing (not all-comers with sarcoma) and include a high proportion with multiple primaries. Small numbers for rare genes mean wide confidence intervals. Some gene-specific findings should be interpreted cautiously and need replication. Associations, especially for genes not classically linked to sarcoma (e.g., BRIP1, CHEK2, CDH1), require validation in larger, unselected cohorts and ideally supported by tumor genomics.
What’s Next
Prospective, unselected sarcoma cohorts with standardized testing will help refine prevalence estimates and gene-specific risks. Likewise, paired tumor-normal sequencing can clarify mechanism (e.g., second hits, mutational signatures) and therapeutic relevance. These data support discussion about expanding germline testing recommendations beyond current criteria focused on classic syndromes or young age.
Takeaway and call to action: Inherited cancer predisposition is common in people with sarcoma and spans more genes, and ages, than many clinicians might expect. Multigene germline testing can inform treatment choices, guide radiation and surgical decisions, streamline surveillance, and enable preventive care for families. If you care for patients with sarcoma, consider building routine germline testing and genetic counseling into your care pathway.
TL;DR
• 26.2% of people with sarcoma had a germline pathogenic variant vs 10.5% of matched controls (OR 3.05).
• Age at sarcoma diagnosis did not predict germline status; gPVs were found across all age groups.
• 6.8% of people with sarcoma had potential therapeutically actionable findings (e.g., PARP inhibitor or immunotherapy eligibility).
• Results support expanding multigene germline testing for all patients with sarcoma.
Link to full article: https://pubmed.ncbi.nlm.nih.gov/41092038/



